Manufactory
Manufactory
Management
The pharmaceutical company is one of the leading pharmaceutical companies in the Republic of Yemen and is characterized by its attraction to the local market. With many specialized and non-specialized medicinal products, as it ranks first in achieving drug adequacy. And the pharmaceutical company has a lot of production lines that achieve the company's high production capacity competing with many pharmaceutical companies.

The pharmaceutical company follows the rules and practices of good pharmaceutical manufacturing GMP by :
1
Ventilation System
Special ventilation system characterized by :

2
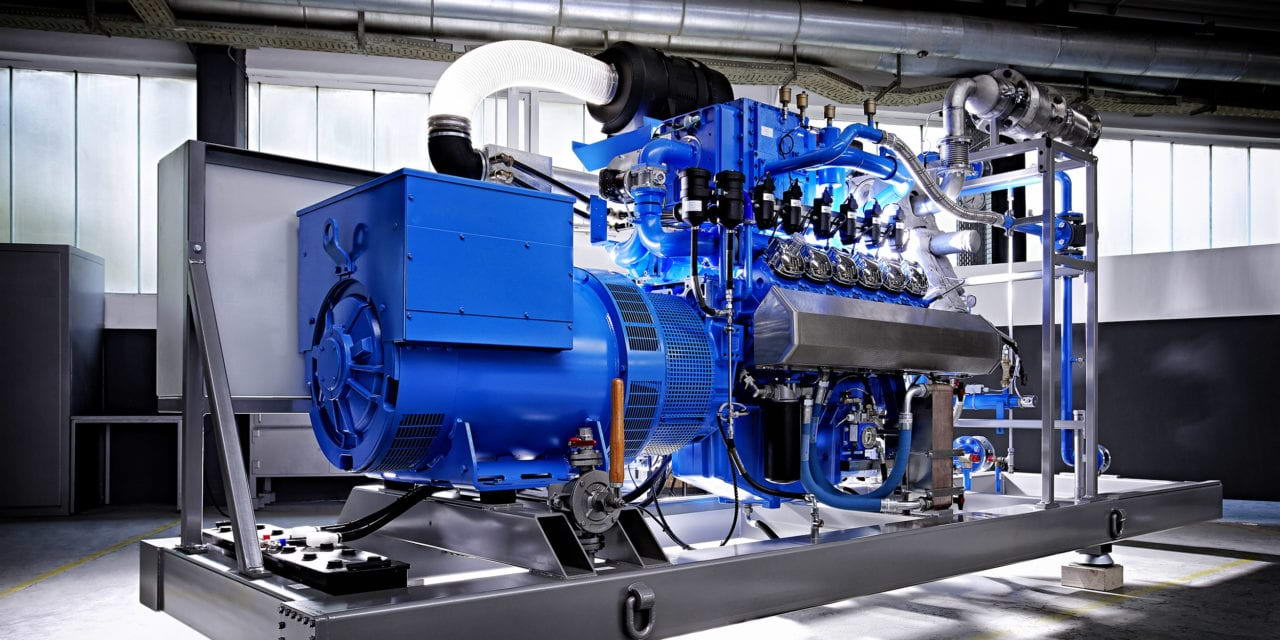
Electrical System
Special electrical system consists of :
3
Water System
It will consist of water treatment unit and exchange of ions, which are working to prepare pure water completely drinkable This unit is characterized by :

4
Fire Alarm System

5
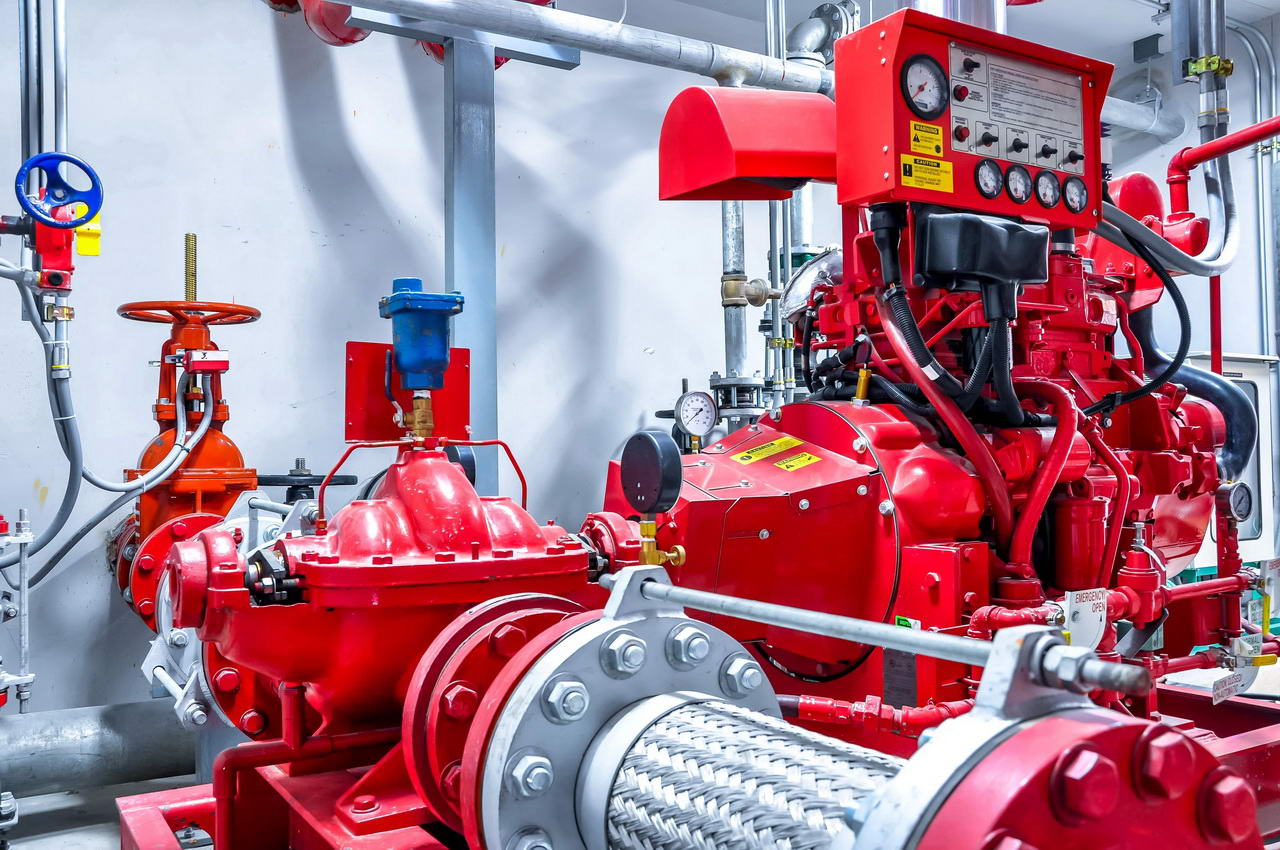
Fire Protection System
In case of occurrence
6
Compressed Air System
Free of oil and filter provided to all production units and service units

7
Maintenance & Preventive Maintenance System
Special ventilation system characterized by :

8
Conservation & Control System
A system of conservation and control of the environment through :

9
Authentication System
Special authentication system Where documents are controlled by :


